Ques.11. When a pentavalent impurity is added to a pure semiconductor it becomes______
- Intrinsic
- n-type
- p-type
- None of the above
Answer.2. n-type Explanation:- Doping:- One way to raise conductivity is by doping. This means adding impurity atoms to a pure tetravalent crystal (intrinsic crystal). A doped material is called an extrinsic semiconductor. Impurity atoms added to the semiconductor change the thermal equilibrium density of electrons and holes. In the case of silicon, the appropriate impurities are elements from the 5th and 3rd columns of the periodic table, e.g. such as phosphorus and boron. By doping, two types of semiconductors may be produced. Generally, there are two types of extrinsic semiconductor First of them are n-type semiconductors with a pentavalent (phosphorus) impurity where the “n” stands for negative because their conduction is due to a transfer of excess electrons. A pentavalent atom, the one that has five valence electrons is called a donor. Each donor produces one free electron in a silicon crystal. In an n-type semiconductor, the free electrons are the majority carriers, while the holes are the minority carriers because the free electrons outnumber the holes. N-type semiconductors are created by doping an intrinsic semiconductor with donor impurities. In an n-type semiconductor, the Fermi energy level is greater than that of the intrinsic semiconductor and lies closer to the conduction band than the valence band. As shown in the figure, each of the four out of five valency electrons of impurity says of Arsenic enters into covalent bonds with Germanium, while the fifth valence electron is set free to move from one atom to the other. The impurity is called donor impurity as it donates an electron and the crystal is called N-type semiconductor. A small amount of Arsenic (impurity) injects billions of free electrons into Germanium thus increasing its conductivity enormously. In N-type semiconductor, the major carriers of charge are the electrons and holes are minority carriers. This is because when donor atoms are added to a semiconductor, the extra free electrons give the semiconductor a greater number of free electrons than it would normally have And, unlike, the electrons that are freed because of thermal agitation, donor electrons do not produce holes. As a result, the current carriers in a semiconductor doped with pentavalent impurities are primarily negative electrons. The impurity atom has five valence electrons. After donating one electron, it is left with + I excess charge. It then becomes a positively charged immobile ion. It is immobile because it is held tightly in the crystal by the four covalent bonds. It is important to understand that in N-type semiconductors, although electrons (negative charges) are the majority carriers, but the semiconductor doped with impurity remains electrically neutral. Free electrons and holes are generated in pairs due to thermal energy and negative charge of electrons donated by impurity atoms is exactly balanced by the positive charge of the immobile ions.
![]()
Ques.12. Addition of pentavalent impurity to semiconductors creates many______
- Free Electrons
- Holes
- Valence electrons
- Bound electrons
Answer.1. Free Electrons Explanation:- Pentavalent-Impurity The n-type semiconductor is formed by doping a pure silicon or germanium crystal with a material having five valence electrons. Antimony, arsenic, and phosphorous are examples of pentavalent materials. If arsenic in very small quantity is added to a silicon crystal, four out of five valence electrons will form covalent bonds with silicon atoms with one electron left free. Thus, for each arsenic atom, there will be one free electron. Although the percentage of arsenic added is very small, the number of atoms being very large, a huge amount of free electrons will be available in the n-type semiconductor. These electrons being free (not taking part in any covalent bonding) are loosely bound to their parent atom and are free to conduct electricity. Fig. shows impurity atoms of antimony having five valence electrons forming covalent bonds with germanium having four valence electrons. There is one extra electron for each impurity atom added. This extra electron is not a part of any covalent bond and is called a free electron. This free electron has been shown out of the orbit. Each antimony atom is making covalent bonds with four neighbouring germanium atoms. Each bond has one electron belonging to germanium atom and one electron belonging to antimony atom. Each antimony atom will make four covalent bonds with four germanium atoms. By sharing of electrons in the covalent bonds all the atoms will satisfy their need to have all the eight positions filled in their outermost orbit, i.e., their valence shells. It may be noted that the n-type semiconductor thus formed remains electrically neutral, i.e., neither positively charged nor negatively charged. This is because the total number of electrons including the free electrons is equal to the total number of protons in the nuclei of the atoms.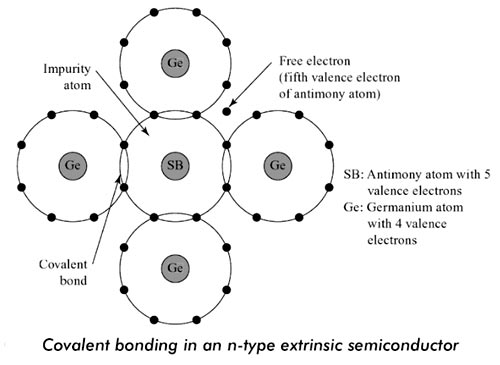
Ques.13. A pentavalent impurity has________
- 3 Valence electrons
- 6 Valence electrons
- 4 Valence electrons
- 5 Valence electrons
Answer.4. 5 Valence electrons Explanation:- The n-type semiconductors with a pentavalent (phosphorus) impurity where the “n” stands for negative because their conduction is due to a transfer of excess electrons. A pentavalent atom, the one that has five valence electrons is called a donor. Each donor produces one free electron in a silicon crystal. In an n-type semiconductor, the free electrons are the majority carriers, while the holes are the minority carriers because the free electrons outnumber the holes.
Ques.14. An n-type semiconductor is
- Positively charged
- Electrically neutral
- Negatively charged
- None of the above
Answer.2. Electrically neutral Explanation:- Charge on n-type and p-type Semiconductors In an n-type semiconductor, current conduction is due to an excess of electrons whereas, in a p-type semiconductor, conduction is by holes. We may think that n-type material has a net negative charge and p-type a net positive charge. But this conclusion is wrong. It is true that n-type semiconductor has an excess of electrons but these extra electrons were supplied by the atoms of donor impurity and each atom of donor impurity is electrically neutral. When the impurity atom is added, the term “excess electrons” refers to excess with regard to the number of electrons needed to fill the covalent bonds in the semiconductor crystal. The extra electrons are free electrons and increase the conductivity of the semiconductor. The situation with regard to the p-type semiconductor is also similar. It follows, therefore, that n-type, as well as p-type semiconductor, is electrically neutral. The n-type semiconductor thus formed remains electrically neutral, i.e., neither positively charged nor negatively charged. This is because the total number of electrons including the free electrons is equal to the total number of protons in the nuclei of the atoms.
Ques.15. A trivalent impurity has_____
- 3 Valence electrons
- 5 valence electrons
- 6 valence electrons
- 4 valence electrons
Answer.1. 3 Valence electrons Explanation:- N-type Semiconductor The semiconductors with a trivalent (boron) impurity have the hole type of conduction or deficit conduction by transfer from atom to atom of electrons into available holes. A semiconductor in which the conduction is due to holes referred to as a p-type semiconductor. Here, p stands for positive because of the carriers acting like positive charges, for the hole travels in a direction opposite to that of the electrons filling it. A trivalent atom, the one that has three valence electrons is called an acceptor or recipient. Each acceptor produces one hole in a silicon crystal. In a p-type semiconductor, the holes are the majority carriers, while the free electrons are the minority carriers because of the holes outnumber the free electrons.
Ques.16. Addition of trivalent impurity to a pure semiconductor creates many________
- Free Electrons
- Valence electrons
- Holes
- Bound electrons
Answer.3. Holes Explanation:- P-Type material is formed when silicon or germanium crystal is doped with (added with) a small percentage of trivalent impurity material like boron, gallium or indium. When covalent bonds are formed between boron having three valence electrons with silicon having four valence electrons, there will be the shortage of one electron in the covalent bonds. This is represented by an empty space in the covalent bonds and is called a hole as shown in Fig. There will be one hole corresponding to each of the impurity atoms taking part in forming covalent bonds. This makes seven out of eight positions filled. One position is left vacant which we called a hole. Therefore, for each gallium atom added, one hole will be available in the silicon crystal. Though each gallium atom added provides one hole, yet an extremely small amount of Boron impurity provides enough atoms to supply millions of holes. Since this type of extrinsic semiconductor provides a large number of holes, it is called p-type semiconductor (p stands for the positive charge on a hole). Similar to n-type material, p-type material is also electrically neutral. The total number of electrons in the orbits is equal to the total number of protons in the nucleus of the atoms. Although the amount of impurity material added is small, the total number of atoms being large produce a large number of holes in the crystal. Thus a p-type material will have plenty of holes and an n-type material will have plenty of free electrons. Electrons and holes constitute charge carriers. In a p-type material, the holes are the majority charge carriers. When the temperature is raised there will be the creation of free electrons and holes. These thermally generated electrons will be the minority charge carriers because of their being small in numbers.![]()
Ques.17. A hole in a semiconductor is defined as_________
- A free electrons
- The incomplete part of an electrons pair bond
- A free proton
- A free neutron
Answer.2. The incomplete part of an electrons pair bond Explanation:- It has been observed that when a trivalent impurity is added to a pure semiconductor, it displaces some of the atoms. Fig. shows the structure of silicon crystal containing an iridium atom at the central position. The 3 valence electrons, of an indium atom, form 3 covalent bonds by sharing one electron with the electrons of neighboring atoms. However, the fourth covalent bond is incomplete. The absence of the electron in the covalent bond is represented by the small circle in Fig. and such an incomplete covalent bond is called a hole. A hole is not a vacancy. A vacancy indicates a missing atom, whereas a hole denotes a missing electron. This hole acts in many respects as a positive charge because it will attract and capture any electron in the immediate vicinity, as presented in Fig. Occasionally, a free electron will approach a hole, fill its attraction, and fall into it. This merging of a free electron and a hole is called recombination. Now, the iridium atom seeks its surrounding atoms so as to acquire the 4th electron, to complete the covalent bond. Thus, an electron which is in a favorable position is captured by an indium atom. After doing this, the indium atom becomes an immobile ion. ![]()
Ques.18. A pentavalent impurity is called_______
- Donor impurity
- Acceptor impurity
- Ionic impurity
- None of the above
Answer.1. Donor impurity Explanation:- Pentavalent impurities: The elements whose atom has five valance electrons are called pentavalent impurities e.g. As, P. Sb etc. These impurities are also called donor impurities because they donate an extra free electron.
Ques.19. The magnitude of the charge of a hole is
- Zero
- Equal to that of a proton
- Equal to that of an electron
- Equal to that of a neutron
Answer.1. Equal to that of an electron Explanation:- Heavily doped semiconductors tend to behave in a similar fashion as a metal having a large number of charge carriers, i.e., electron-hole pairs. It must be remembered that the electrons act as charge carriers in the conduction band whereas the positive holes act as the charge carriers in the valence band. Both of these charge carriers are equally responsible for conduction in their respective bands. When an electron is missing from this structure the bond has one electron less thus termed as a hole in the bond. The atom with one electron missing from its outermost orbit may be termed as +ve charged ion Similarly, the structure may have an excess electron, thus simulating the condition of -ve charged ion. The holes (vacancies of electrons) may move from ion to ion in the crystal and produce the effect of motion of +ve charge (the hole). Each hole has a charge equal to that of an electron, in magnitude.
Ques.20. As a general rule, holes are found only in_______
- Metals
- Semiconductor
- Insulator
- Resistance materials
Answer.2. Semiconductor Explanation:- The electrons and holes formed by the addition of the donor and acceptor impurities are called majority carriers. As the name implies, these carriers are responsible for most of the current in the extrinsic semiconductor material and lower the resistivity considerably. In addition to the majority carriers, there are a small number of free electrons in p-type material and a small number of free holes in n-type material, produced by thermal agitation of the atoms in the crystal as mentioned previously. These electrons and holes are also available to carry a current and are called minority carriers.



