Which of the following has only covalent bond between the atoms?
Which of the following has only covalent bond between the atoms?
Right Answer is:
HCl
SOLUTION
Covalent bonds are formed by sharing electrons between non-metals to reach a stable valence configuration. The formation of a bond between H and Cl to give an HCI molecule can be represented as
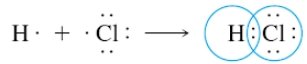
As the two atoms approach each other, unpaired electrons on each atom pair up to form a covalent bond. The pair of electrons is shared by the two atoms. Each atom then acquires a noble-gas configuration of electrons, the H atom having two electrons about it (as in He), and the Cl atom having eight valence electrons about it (,as in Ar).
