21. _______ is a hard solder.
- Copper-zinc
- Tin-lead
- Tin-zinc
- Tin-silver-lead
Answer.1. Copper-zinc
Explanation:-
SOLDER:– Solder is an alloy of metals with a low melting point Aloy means a mixture of two or more metals in a certain proportion to give desired properties. Solders are of two types
- Hard solder
- Soft solder
Hard Solder- The melting point of hard solder is high. Due to this, it is used extensively It is used for soldering metals having high melting points like Steel, Iron, and Brass, etc.
Hard solder is of two types
- Spelter solder
- Silver solder
Spelter Solder
This is a hard solder. It consists of the following metals-
⇒ Copper and Zinc alloyed solder— Which has 50% copper and 50% Zinc or Copper 67% and Zinc 33%
⇒ Copper, Zinc, and Tin alloyed solder — Which has 50% copper, 37% Zinc, and 13% Tin.
⇒ In this Copper, Zinc and Cadmium are alloyed.
Silver Solder
Lead-free solders in commercial use may contain tin, copper, silver, bismuth, indium, zinc, antimony, and traces of other metals.
Soft Solder- There are many different types of solder being used by the industry. Solders are available in various forms that include bars, wires, ingots, and powders. Wire solders are available with or without a flux core.
TIN-LEAD SOLDER
They have good corrosion resistance and can be used for joining most metals. A 40 60 solder means to have 40% tin and 60% lead.
⇒ Alloy solder for electrical and copper wires = Tin 50%, Lead 50%
⇒ Alloy solder for Aluminium wires = Tin 70%, Zinc 25%, Aluminium 3%, and Phosphor tin 2%.
22. The resistivity of a metal is a function of temperature because
- The electron gas density varies with temperature
- None of the above
Answer.3. The amplitude of vibration of the atoms varies with temperature
Explanation:-
Material can be characterized by its resistivity, denoted by ρ, which is independent of size and shape, The resistance of a homogeneous sample of material having resistivity ρ, length l, and uniform cross-sectional area A is given by
$R = \left[ {\frac{m}{{n{e^2}\tau }}} \right]\frac{L}{A}$
Where
m = mass of the electron
n = number of free electrons per unit volume
τ = Average relaxation time
or
$R = \rho \frac{L}{A}$
Resistivity is defined as the resistance of a conductor of unit length and unit area of cross-section. Its SI unit is Ohm-m (Ω-m).
Comparing
$\rho = \left[ {\frac{m}{{n{e^2}\tau }}} \right]$
We know that the resistivity of the metals is due to the scattering of the conduction electrons. The resistivity of the metal is divided into two components namely ideal and residual resistivity.
When the electrons are scattered by lattice vibration, i.e., phonons free from all defects give rise to ideal resistivity ρph. The ideal resistivity is temperature-dependent. On the other hand, when the electrons are scattered by the presence of the impurities and imperfection like dislocation vacancies, grain growth, and boundaries, give rise to residual resistivity ρr . The residual resistivity is independent of temperature and hence, the remaining does not need to be zero even at T = 0 K. Therefore, the resistivity of a metal is
ρ = ρph + ρr
At low temperatures, the amplitude of lattice vibration is very small and hence, the scattered electrons are also very less. Thus, it results in a larger τph, and hence, the ρph is almost equal to zero. Therefore, the total resistivity of the metal is equal to
ρ = ρr
On the other hand, when the temperature increases, the amplitude of lattice vibration increases, and hence, the scattering of electrons by the lattice also increases. Therefore, the resistivity of lattice vibration is added to total resistivity (ρ = ρph + ρr), and hence, a linear increase in resistivity with increases in temperature is observed at low temperatures.
At high temperatures, the scattering effects due to phonons overcome the effect due to scattering and impurities which give rise to the resistivity which depends on lattice vibration only. As a result, at the higher temperature, the resistivity of metals which depends purely on lattice vibrations varies exponentially than at lower temperature. Similarly, at low temperature, the resistivity of metal depends on lattice vibration and residual resistivity and hence, shows a linear variation with temperature.
In a pure metal, the scattering of electrons takes place only due to lattice vibrations. But in the case of impure metals, the scattering of electrons is both due to the lattice vibrations and impurity scattering.
23. The minority carrier concentration is largely a function of
- Forward biasing voltage
- Temperature
Answer.4. Temperature
Explanation:-
The majority carriers in an n-type semiconductor are free electrons acquired by the doping process and the minority carriers are holes produced by thermally generated electron-hole pairs. These electron-hole pairs are thermally produced because the electron has acquired enough energy from external heat to break away from its atom.
A free-electron will eventually lose energy and fall back into a hole. This is called recombination. Electron-hole pairs are continuously being thermally generated so there are always free electrons in the material.
The minority carriers are thermally produced and they exist only for a short time after which they recombine and neutralize each other. In the meantime, other minority carriers have been produced and this process goes on and on. The number of these electron-hole pairs that exist at any one time depends upon the temperature.
24. The structure sensitive property of a superconductor is
- Critical magnetic field
- Critical current density
- Transition temperature
- Critical electric field
Answer.2. Critical Current density
Explanation
Superconductivity is a phenomenon in which certain metals, alloys, and ceramics conduct electricity without resistance when it is cooled below a certain temperature called the critical temperature.
Critical current density Jc Highest current density that can flow through a superconducting material without driving it normally.
When the current density through a superconducting sample exceeds a critical value JC. the superconducting state is found to disappear in the sample. This happens because, the current through the superconductor itself generates a magnetic field, and at a sufficiently high current density the magnetic field will start exceeding the critical magnetic field HC thereby making the superconducting state disappear in the material.
Hence, the critical current density can be defined as the maximum current that can be permitted in a superconducting material without destroying its superconductivity state. The critical current density is a function of temperature, i.e., the colder the temperature for a superconductor the more is the current it can carry.
The critical temperature is the least structure sensitive and critical current density is the most structure-sensitive.
25. Which of the following materials is used for making coils of standard resistances?
- Copper
- Nichrome
- Platinum
- Manganin
Answer.4. Manganin
Explanation
Due to high resistivity and low-temperature coefficient of resistance, manganin wire (Cu − 84% + Mn — 12% + Ni — 4%) is used in the preparation of standard resistances.
26. Spark plug makes use of which of the following materials for insulation?
- Slate
- Asbestos
- Glass
Answer.1. Porcelain
Explanation
The term ceramic comes from the Greek word for “pottery.” It is used to describe a broad range of materials that include glass, enamel, concrete, cement, pottery, brick, porcelain, and chinaware. This class of materials is so broad that it is often easier to define ceramics as all solid materials except metals and their alloys that are made by the high-temperature processing of inorganic raw materials.
Ceramics can be either crystalline or glasslike. They can be either pure, single-phase materials or mixtures of two or more discrete substances. Most ceramics are polycrystalline materials, with abrupt changes in crystal orientation or composition across each grain in the structure. Ceramics can also make excellent insulators, such as the glass-ceramics (porcelain) used in spark plugs.
27. An H.R.C. fuse is
- A wire of platinum
- A ceramic body having metal and caps
- A ceramic tube having carbon rod inside it
Answer.3. A ceramic body having metal and caps
HRC Fuses
High rupturing capacity (HRC) Fuse links provide complete protection to cables, switchgear, control gear, and other equipment by limiting the current, both in magnitude and in the time duration, that can pass through these devices in the circuit. The rapid operation in the event of a fault limits the let-through current and energy, thus minimizing the electromagnetic and thermal stresses on the electrical apparatus.
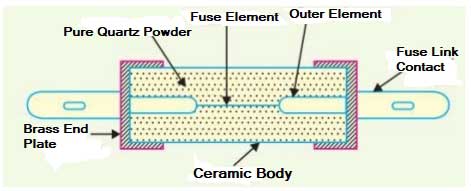
These type of fuses comprises a high-grade ceramic body within which fuse elements are placed and welded to the end plates. The assembly is filled with dry granular quartz sand, plaster of Paris, quartz, chalk, marble, dust and cooling mediums etc. In the event of flow of high short circuit current, this quartz sand solidifies forming high resistance glass in the arc path to ensure effectively are quenching in optimum time. The fuse elements are the non-deteriorating type which means that they maintain their characteristics over the long service period.
28. For germanium the forbidden energy gap is
- 0.15 eV
- 0.25 eV
- 0.5eV
Answer.2. 0.7 eV
Explanation
The difference between the highest valence band and the lowest conduction band is called the energy band gap or the energy gap.
The forbidden energy gap for germanium is 0. 7 em and for silicon 1.1 em.
29. High resistivity materials are used in
- Precision instruments
- Heating elements
- Motor starters
- All of the above
Answer.4. All of the above
Explanation
High Resistivity Materials
These are basically alloys of different metals and based on applications, they can be divided into the following three groups:
- Shunts in electrical measuring instruments
- Wire-wound precision resistances
- Resistance boxes
- Starters for electric motors
- Loading rheostats
- Heating elements for heaters, ovens, starters etc.
- Filaments for incandescent lamps.
(i) The materials which are used in precision electrical measuring instruments for the construction of standard resistances and resistance boxes.
(ii) The materials which are used for the construction of resistance elements such as rheostats and control devices.
(iii) The materials which are used for the construction of furnaces. healing devices and loading rheostats.
The materials which are used for the construction of standard resistances and resistance boxes for precision electrical measuring instruments should have the stability of resistance with respect to time and temperature. They must have a low value of temperature coefficient of resistivity and low thermoelectric emf, The material commonly used for such applications is manganin (Cu 86%, Mn and Ni 2%).
The materials which are commonly used for making rheostats and control device may have comparatively higher temperature coefficient 01′ resistance and higher thermoelectric emf but should have low cost and permissible working temperature since these are used in bulk quantity, Constantan (Cu and Ni 40%) is commonly used for such applications. In many applications, nickel, an alloy of copper-nickel and zinc-containing less amount of nickel is used.
The materials which are used as heating elements in furnaces should have a high melting point and must withstand corrosion. The temperature coefficient of resistivity is of less importance in such materials. Platinum is used in laboratory-type electrical furnaces since it can be operated at elevated temperatures of about 1300°C and is an incorrodible material. Nichrome is extensively used for high-temperature applications.
Eureka is used for making starter and field regulator resistances: wires for resistance boxes and thermocouples.
30. Which of the following materials does not have covalent bonds?
- Organic polymers
- Silicon
- Metals
- All of the above
Answer.3. Metals
Explanation
- Covalent bonds form between non-metal atoms.
- The atoms share some of their outer electrons so that each atom has a full outer shell Of electrons.
- A covalent bond is a shared pair of electrons.



