11. The conductivity of a conductor can be increased by
- Decrease with temperature
- Increasing with temperature
- Decreasing with vibration
- Increasing with vibration
Answer.1. Decrease with temperature
Explanation:-
In metallic conduction, the temperature has the effect of decreasing the conductance, i.e., the conductance of a metallic conductor decreases with an increase in temperature. Metallic conductance becomes pronounced at low temperatures. This is because, at low temperatures, the kernels of the atoms have the least vibrations and offer the least resistance to the flow of electrons.
12. The kinetic energy of a bounded electron is
- Greater than that of unbounded electron
Answer.4. Less than that of unbounded electron
Explanation:-
Lone pair or Unbonded pair:- A pair of electrons in a molecule that occupies an atomic orbital but does not participate in bonding. Lone pair electrons are not shared between atoms. molecular orbital.
Bond pair refers to those electrons which are present between two atoms and that are responsible for the formation of a bond (a linkage ) between two atoms of a different kind like HCl or the same kind like H2. Electrons that are included in bond pair have minimum repulsion it means they don’t resist each other rather than they overcome the severe repulsion between them and stay together like a pair for the sake of bond formation
As we know lone pair is the electron pair that doesn’t take part in bond formation. It means there is no overlapping between the lobes of the orbital which can make it unstable whereas in shared electron pair there is overlapping that takes place so it is stable.
And one thing we know that greater the stability there will be less energy and vice-versa.
13. Mercury as an electric contact material is
- A liquid
- A metal
- A metal liquid
- A gas
Answer.3. A metal liquid
Explanation:-
Most metals are hard solids at normal temperatures. The exception is mercury, which is a shiny, silvery liquid, It does not turn solid, or freeze until it gets below —39°C (—38.2° F), which is twice as cold as inside a household freezer.
The outermost orbital of mercury is 6s. 6s is strongly bound to the nucleus. Therefore it is difficult to remove the outer electrons unlike in other metals. Hence mercury cannot easily form bonds with other elements. This is the reason why mercury exists in a liquid state.
14. Superconductivity can be destroyed by
- Application of magnetic field
- Reducing temperatures
- All of the above
Answer.2. Application of magnetic field
Explanation:-
Superconductivity is a phenomenon in which certain metals, alloys, and ceramics conduct electricity without resistance when it is cooled below a certain temperature called the critical temperature.
A superconductor is a material that loses all its resistance (offers zero resistance) to the flow of electric current when it is cooled below a certain temperature called the critical temperature or transition temperature TC. Examples: Mercury (Hg), Zinc (Zn), Vanadium (V), Tin (Sn) and Niobium (Nb).
Properties of Superconductors
Few important properties of superconductors are explained in brief in this section.
(i) Critical temperature TC (Transition Temperature)
The electrical resistance of the superconducting materials becomes zero below the transition temperature (Tc). This property is called the defining property of superconductors. The figure shows the variation of electrical resistance with respect to the temperature for the normal conductor and a superconductor. From the figure, it is noted that the resistance falls to zero at the transition temperature.
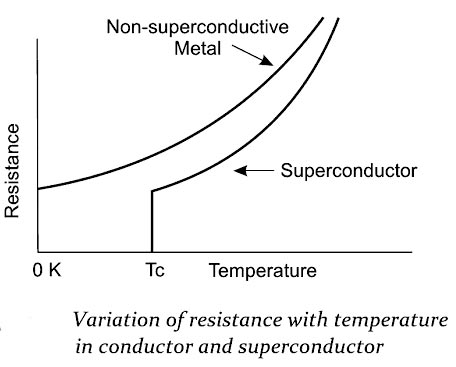
Note:- Good electrical conductors such as silver (Ag), Gold (Au) and copper (Cu) are not good superconductors because the resistivity of these conductors at low temperatures is limited to the low resistivity.
Similarly, good superconducting materials like Zn and Pb are not good electrical conductors.
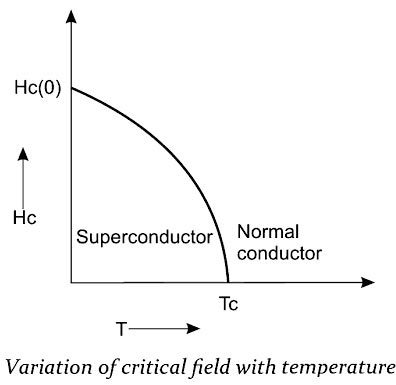
(ii) Magnetic field effect
If a sufficiently strong magnetic field is applied to a superconductor at any temperature below its critical temperature TC, the superconductor is found to undergo a transition from the superconducting state to the normal state.
This minimum magnetic field required to destroy the superconducting state is called the critical magnetic field HC.
15. ______ has the best damping properties.
- High-speed steel
- Cast iron
- Mild steel
- Diamond
Answer.2. Cast Iron
Explanation:-
A damping coefficient is a material property that indicates whether a material will bounce back or return energy to a system. If the bounce is caused by an unwanted vibration or shock, a high damping coefficient in the material will diminish the response. It will swallow the energy and reduce the undesired reaction. Such measures are required when evaluating material and system responses to dynamic loading conditions.
Materials with high damping coefficients are used in applications of shock absorption, vibration control, noise reduction and dissipating increased heat. Engineers use damping coefficients to compare materials to see which will be the best one for the application. This number describes the behavior of the material in a damped system.
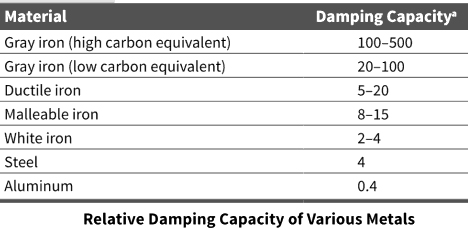
Gray iron contains carbon in the form of flakes. The flake-like shape of graphite in gray iron exerts a dominant influence on its mechanical properties. As a result, gray iron exhibits no elastic behavior but excellent damping characteristics and fails in tension without significant plastic deformation. The presence of graphite flakes Also gives gray iron excellent machinability and self-lubricating properties
The flake graphite provides gray iron with unique properties such as excellent machinability at hardness levels that produce superior wear-resisting characteristics, the ability to resist galling, and excellent vibration damping.
Gray irons provide ten times the damping of low-carbon steels while ductile irons provide three times as much damping.
16. In a system, the damping coefficient is — 2. The system response will be
- Undamped
- Oscillations with decreasing magnitude
- Oscillations with increasing magnitude
- Critically damped
Answer.3. Oscillations with increasing magnitude
Explanation:-
The damping coefficient is a material property that indicates whether the material will bounce back or return energy to a system. In the case of negative damping, the damping force does positive work on the system and oscillation with increasing magnitude.
17. Selenium is _____ semiconductor
- Extrinsic
- Intrinsic
- N-type
- P-type
Answer.2. Intrinsic
Explanation:-
A material that is naturally semi-conducting in the pure state is known as an intrinsic semiconductor. A semiconductor whose conductivity results from the presence of impurity centers or of imperfections is called an extrinsic semiconductor.
An intrinsic semiconductor such as silicon, selenium, or germanium can therefore conduct an electric current under certain conditions. Semiconductors such as germanium, silicon, selenium, and a variety of complex compounds are manufactured in very pure crystalline forms and have very small levels of impurities added (doping).
18. The uses of selenium are
- Photocell
- Rectifier
- Stainless steel
- All of the above
Answer.4. All of the above
Explanation:-
The selenium is used in Blasting caps, Chromium Plating, Stainless steel, Photocell, and Rectifier
Stainless Steel
Selenium is used in stainless steel to improve casting, forging, and machinability without reducing corrosion resistance or the hot-forging and cold-working properties. The selenium content of casting steel alloys ranges from 0.01 to 0.05 percent, forging steels from 0.18 to 0.22 percent, and free-machining steels from 0.05 to as much as 0.35 percent.
Rectifier
Selenium has been used in rectifiers for many years to rectify (change) alternating current electricity to direct current. Selenium rectifiers are extensively used in electroplating, welding, dc motor operation, battery chargers, magnetic coils, arc lamps, induction braking, and voltage regulators.
Photocell
Selenium also is used in the photoelectric cell. selenium cell. One Side of the selenium component in each device is coated with functionally different materials; an electrically conducting translucent film of metal, such as gold foil, is used in the photocell, and an electrically conducting opaque layer of metal. When light falls on the film a potential difference due to the PHOTOVOLTAIC EFFECT is set up between the selenium and the gold.
Selenium photoelectric cells are used in photographic exposure meters, detectors, electric eyes, colorimeters, and pyrometers.
19. The conductors have transport phenomena of electrons due to
- Electric field
- Magnetic field
- Electromagnetic field
- Inductive field
Answer.1. Electric field
Explanation:-
Conductors are substances through which electric charges can flow easily. They are characterized by the presence of a large number of free electrons. The number density of free electrons in a conductor is the same throughout the conductor. This is because free electrons experience repulsive force between them and the conductor allows the movement of free electrons.
In absence of applied potential difference electrons in the conductor have random motion. The average displacement and average velocity are zero. There is no flow of current due to the thermal motion of free electrons in a conductor. When two ends of conductors are joined to a battery then one end is at higher potential and another at lower potential. This produces an electric field inside the conductor from a point of higher to lower potential i.e. E = V/L.
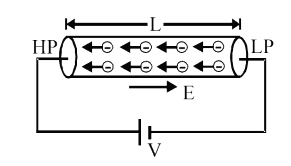
The field exerts an electric force on the free-electron causing acceleration of each electron.
20. Is a negatively charged particle present in an atom.
- Proton
- Neutron
- Electron
- None of the above
Answer.3. Electron
Electrons are negatively charged particles that revolve around the nucleus.



