51. For a particular material, the Hall coefficient was found to be zero. The material is
- Insulator
- Intrinsic semiconductor
- None of the above
Answer.3. Intrinsic semiconductor
Explanation
Measurement of the Hall effect gives an important source of information about conduction mechanisms, especially in semiconductors.
If this current is due to the motion of positive charges (e.g., holes in a p-type semiconductor), then it will result in a positive Hall voltage.
In the case of intrinsic semiconductors, the Hall voltage will be zero because of equal concentrations of electrons and holes. Thus the Hall effect can be used to identify the type of a semiconductor as well as its charge density.
52. The current due to electron flow in the conduction band is _____the hole current in the valence band.
- Equal to
- Less than
- Greater than
- Any of the above
Answer.3. Greater than
Explanation
Charge carriers in a semiconductor can be negative, positive, or both. When an electron moves from the valence band into the conduction band, it leaves behind a vacant site, called a hole, in the otherwise filled valence band. This hole (electron-deficient site) acts as a charge carrier in the sense that a free electron from a nearby site can transfer into the hole. Whenever an electron does so, it creates a new hole at the site it abandoned. Therefore, the net effect can be viewed as the hole migrating through the material in the direction opposite the direction of electron movement. The hole behaves as if it were a particle with a positive charge +e.
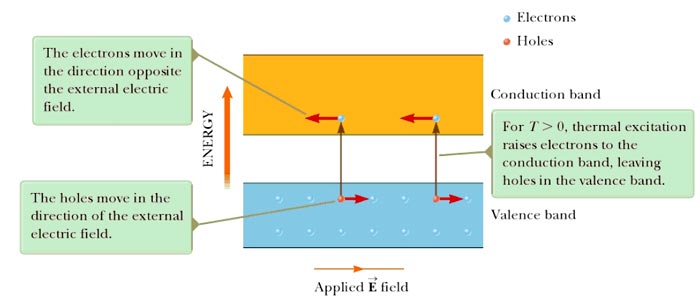
It should be noted that the electron moves more rapidly to the positive terminal than the hole moves toward the negative terminal since the probability of an electron having the energy required to move to an empty state in the conduction band (which is almost empty) is much greater than the probability of an electron having the energy required to move to an empty state in the valence band (which is almost filled). The electron mobility is often greater than hole mobility because quite often the electron effective mass is smaller than hole effective mass. Thus the current due to electron flow in the conduction band is greater than the hole current in silicon. However, the net current is small. and hence the material is a semiconductor.
53. A perfect conductor has
- Zero conductivity
- Infinite conductivity
- None of the above
Answer.3. Infinite conductivity
Explanation
A perfect conductor is one that has the same potential everywhere on it. Such surfaces are called equipotential surfaces. Hence the potential difference between the ends of a perfect conductor is zero. It may be noted that a perfect conductor has infinite conductivity.
This statement is based on Ohm’s law which states that Temperature remaining constant, the current flowing through a conductor is directly proportional to the potential difference existing between its ends.
According to Ohm’s Law
I = (V1 − V2)/R = G(V1 − V2)
Where
I = current flowing through the conductor
(V1 − V2) = Potential difference across the conductor
G = Conductance of the conductor
G = I/(V1 − V2)
For perfect conductor (V1 − V2) = 0
G = I/0 = ∞
Thus proved the perfect conductor has infinite conductivity.
54. The metal having the lowest temperature coefficient of resistance is
- Gold
- Copper
- Aluminum
- Iron
Answer.1. Gold
Explanation
⇒ The temperature coefficient of gold is 34 x 10-4
⇒ The temperature coefficient of copper is 40 x 10-4
⇒ The temperature coefficient of Aluminum is 43 x 10-4
⇒ The temperature coefficient of Iron is 56 x 10-4
Thus gold has the least temperature coefficient of resistance
55. The total number of crystal systems is
- 7
- 2
- 4
- 5
Answer.1. 7
Explanation
Crystal systems:- Most minerals are crystalline as they develop crystal forms, geometrical bodies which are specific to and typical of that mineral. All crystal forms can be assigned to seven crystal systems (cubic, tetragonal, hexagonal, trigonal, orthorhombic, monoclinic, triclinic). These systems are differentiated according to the axes of the crystals, the angles at which the axes intersect, and the symmetry.
56. _____ are used to change the amount of resistance in the circuit
- Variable resistor
- All of the above
Answer.4. All of the above
Explanation
Variable resistors are used to vary or change the amount of resistance in a circuit. Variable resistors are
called potentiometers or rheostats. Potentiometers generally consist of carbon-composition resistance elements,
while the resistance element in a rheostat is usually made of resistance wire.
57. Superconductors are becoming popular for use in
- Manufacture of bubble memories
- Generating electrostatic field
Answer.3. Generating very strong magnetic field
Explanation
Superconducting Magnets
A superconducting magnet is an electromagnet made from coils of superconducting wire. The basic construction of such superconducting magnets consists of a solenoid wound with superconducting wire, which comprises the fundamental design of every high-frequency NMR magnet. The niobium-titanium alloy (NbTi) embedded in a copper matrix has been used to construct solenoids for field strengths up to 9.4 T (400 MHz).
For superconducting magnets, the wire can conduct much larger electric currents than ordinary wire; hence very strong magnetic fields can be created. Superconducting magnets have a number of advantages over resistive electromagnets. They can achieve an order of magnitude stronger field than ordinary ferromagnetic-core electromagnets, which are limited to fields of around 2 T. The field is generally more stable, resulting in less noise in experimental measurement, and its operation does not require expensive consumption of electrical power and cooling water, as required for the electromagnets.
58. In graphite, bonding is
- Metallic
- Vander Waals
Answer.4. Vander Waals and covalent
Explanation
Covalent Bond
It is also known as a homopolar bond. When Two or more atoms coming close together to share electrons and make a stable structure jointly such that all atoms have eight electrons in the outermost orbit.
Vander Waals bond
Positively charged atomic nuclei, when surrounded by an electron cloud, may create a situation when some kind of electrical imbalance is created in the structure. The result is a weak force of attraction between certain substances. This is the bond which has been called van der Walls’ bond or Residual bond.
Vander Waals bond though not an Independent bond among minerals nevertheless is present in minerals along with other types of bond-like, ionic, and covalent and impart of weak character to the mineral-like in graphite where carbon atoms are covalently bonded to a sheet form but different sheets are joined together by a weak van der Walls’ bond making graphite very soft and friable.
Hence both covalent and Vander Waals bond is present in the graphite.
59. The converse of hardness is known as
- Malleability
- Toughness
- Softness
- None of the above
Answer.3. Softness
Explanation
Hardness:– It is the property of a material that enables it to resist abrasion, indentation, and scratching by a harder body, i.e., hardness enables the material to resist wear due to friction. Softness is the converse of hardness. Typical examples are cast iron, manganese, and chrome steel.
60. The advantages of the carbon material are
- High melting point
- Low Density
- Electrical usability
- All of the above
Answer.4. All of the above
Explanation
Carbon is available in different forms i.e. the diamond with extreme hardness and graphite with grey and soft. Carbon materials used in electrical engineering are manufactured from graphite and other forms of carbon. Graphite is available in nature as a mineral with high content of carbon (upto 95%).
It is crystalline in structure and has a very high melting point (approx. 3900° C). Pure carbon is a semiconductor and has a negative temperature coefficient of resistivity. The conductivity of carbon is slightly less than that of metals and their alloys.
Carbon is extensively used as brushes for electrical machines, carbon electrodes for electric furnaces, electrolytic baths, arc welding, non-wire resisters, arc light, battery cell elements microphone powders, etc.
The brushes made with carbon are almost universally used for current collection from the rotating parts of electrical machines. Carbon and graphite are continuously used for sliding electrical contacts because the use of them as a contact material has several advantages.
⇒ Maintaining its properties under high temperatures. High instantaneous temperatures exist at the point of rubbing contacts. Carbon retains its properties under such conditions and remains solid even above the temperature of 3000°C.
⇒Carbon has a low density and hence it is lighter than several metals, only magnesium is comparable with carbon.
⇒ Carbon cannot be welded to metals under the conditions in which metals weld to one another, like in the heat of an electric arc.



